Coronavirus: US Volunteers to Test First Vaccine

The first
human trial of a vaccine to protect against pandemic coronavirus is starting in
the US later on Monday, according to reports.
A group of
45 healthy volunteers will have the jab, at the Kaiser Permanente research
facility, in Seattle.
The vaccine
cannot cause Covid-19 but contains a harmless genetic code copied from the
virus that causes the disease.
Experts say
it will still take many months to know if this vaccine, or others also in
research, will work.
Scientists
around the world are fast-tracking research.
And this
first human trial, funded by the National Institutes of Health, sidesteps a
check that would normally be conducted - making sure the vaccine can trigger an
immune response in animals.
But the
biotechnology company behind the work, Moderna Therapeutics, says the vaccine
has been made using a tried and tested process.
Dr John
Tregoning, an expert in infectious diseases at Imperial College London, UK,
said: "This vaccine uses pre-existing technology.
"It's
been made to a very high standard, using things that we know are safe to use in
people and those taking part in the trial will be very closely monitored.
"Yes,
this is very fast - but it is a race against the virus, not against each other
as scientists, and it's being done for the benefit of humanity."
Typical
vaccines for viruses, such as measles, are made from a weakened or killed
virus.
But the
mRNA-1273 vaccine is not made from the virus that causes Covid-19.
Instead, it
includes a short segment of genetic code copied from the virus that scientists
have been able to make in a laboratory.
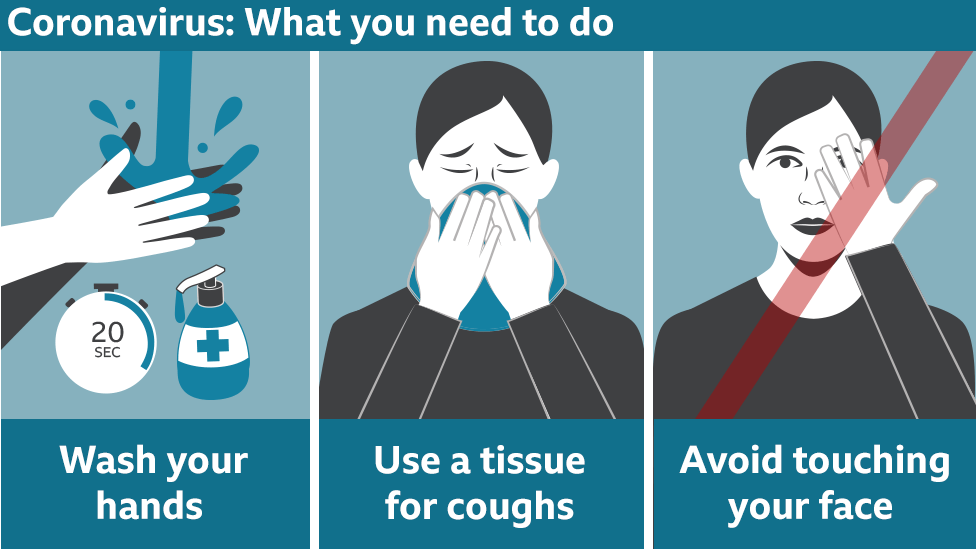
This will
hopefully prime the body's own immune system to fight off the real infection.
The
volunteers will be given different doses of the experimental vaccine.
They will
each be given two jabs in total, 28 days apart, into the upper arm muscle.
But even if
these initial safety tests go well, it could still take up to 18 months for any
potential vaccine to become available for the public.
Meanwhile,
the European Commission has proposed a temporary ban on non-essential travel to
the European Union.
The measure
would initially last for 30 days, and long-term residents in the EU, family
members of EU nationals and diplomats would be exempt as well as cross-border
and healthcare workers and people transporting goods.
The
restriction would initially be a decision for the states part of the Schengen
agreement on free travel between European countries. Non-Schengen members -
including the UK - would be invited to take part.
Latest World
Health Organization (WHO) figures list 164,000 confirmed cases and 6,470
deaths worldwide.
However,
last week it said Europe was now the "epicentre" of the virus and
urged governments to act aggressively to control the spread of Covid-19, the
disease caused by the coronavirus.
Leaders of
the G7 nations are to hold a video conference on Monday to discuss a joint
response to the coronavirus pandemic.
Central
banks around the world, including the US Federal Reserve and those in the UK,
Japan, Canada, and Switzerland have cut interest rates and taken other measures
to try to curb the economic turmoil.
But stock
markets in Asia and Europe still fell and, on Wall St, trading was
temporarily halted after the S&P 500 index dropped 8% on opening. It was
the third time in six days that the session was interrupted.
The EU's
Internal Market Commissioner Thierry Breton said a recession was now expected,
with a 2-2.5% negative growth.
Airlines are
also continuing to slash flights as demand slumps.
FROM .bbc.com/news/health

No comments